Deadly hospital superbug found on the beach of a remote tropical island
Researchers say that this superbug is the first example of a new pathogenic fungus emerging from human-induced global warming.
Remote ecosystems are home to countless curiosities including unique species and microbes that have yet to be detected. This held true for an international team of researchers that discovered Candida auris (C. auris), a deadly hospital superbug, nestled in parts of the Andaman Islands.
The Andaman Islands are located in the middle of the Indian Ocean and are some of the most isolated islands on the planet. Forty-eight samples of sediments and seawater were collected from coastal wetlands, rocky shores, beaches, tidal marshes, and mangrove swamps. Out of eight sites that were sampled, C. auris was found in two places: a salt marsh and a sandy beach.

Mangrove trees rising out of the ocean along the Andaman Islands. Credit: Vyacheslav Argenberg/ Wikimedia Commons. (CC BY-SA 4.0)
A study published by the researchers in mBio states that C. auris is considered “a serious global threat to human health” as it is highly resistant to many antifungal agents and spreads rapidly in hospitals and healthcare systems. The researchers say both the multidrug-susceptible and multidrug-resistant strains of C. auris were found in their samples.
“What is different and particularly scary about C. auris is that it can last on skin and healthcare surfaces, like bedrails and chairs, for a long time—allowing it to spread from person to person in healthcare settings. C. auris is often not killed by commonly used antifungal drugs, making infections difficult to treat and frequently deadly,” the U.S. Centers for Disease Control and Prevention (CDC) states on their website.

A rocky shoreline in the Andaman Islands. Credit: Vyacheslav Argenberg/ Wikimedia Commons. (CC BY-SA 4.0)
While the origins of this superbug are unknown, the study says that the samples from the Andaman Islands indicate C. auris is associated with marine ecosystems and the potential for this fungus to infect humans from nature should be further explored.
WARMER TEMPERATURES FAVOURING CERTAIN FUNGI
Previous studies report that the origins of this pathogen infecting humans are unclear. A study published in mBio in 2019 suggests that C. auris could be native to wetlands and climate change improved the pathogen’s ability to handle warmer waters and changing salt levels.
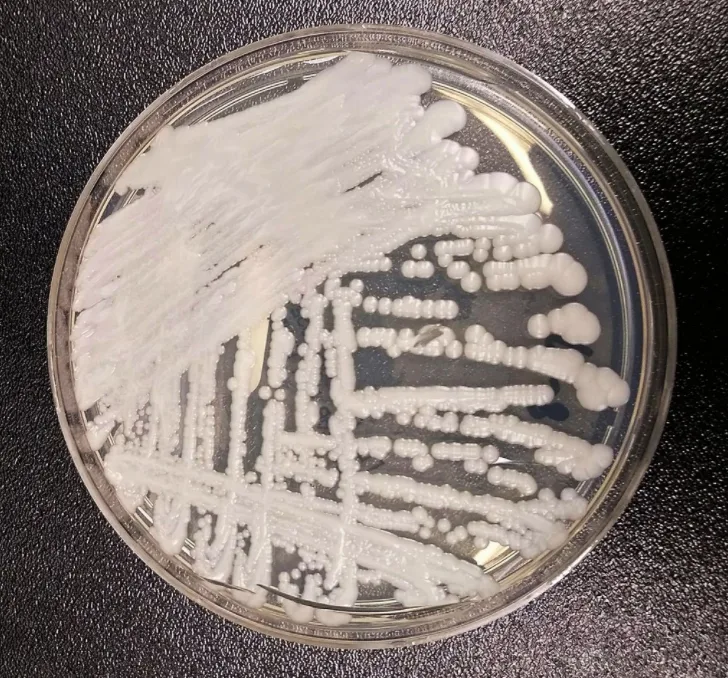
A strain of C. auris cultured in a Petri dish at a CDC laboratory. Credit: CDC/Wikimedia Commons
The 2019 study states that fungal pathogens are rare in mammals because only a few can replicate at 37°C, the normal temperature of the human body. However, these researchers say that warming temperatures could allow for certain fungal strains to become more tolerant to abnormally warm conditions, and could evolve to comfortably live at the internal body temperatures of humans and other species.
While factors including high human population densities, migrations, poor hygiene, pollution, and travel may have played a role in the emergence of C. auris in humans, these researchers say that “C. auris is the first example of a new pathogenic fungus emerging from human-induced global warming.”
“Whether C. auris is the first example of new pathogenic fungi emerging from climate change or whether its origin into the realm of human-pathogenic fungi followed a different trajectory, its emanation stokes worries that humanity may face new diseases from fungal adaptation to hotter climates.”
Thumbnail credit: Dinodia Photo. Corbis Documentary. Getty Images